
01
OLMESARTAN
(Olmesartan Medoxomil)
02
DAPAGLIFLOZIN
(Dapagliflozin Propanediol)
Completed Bilayer Formulation
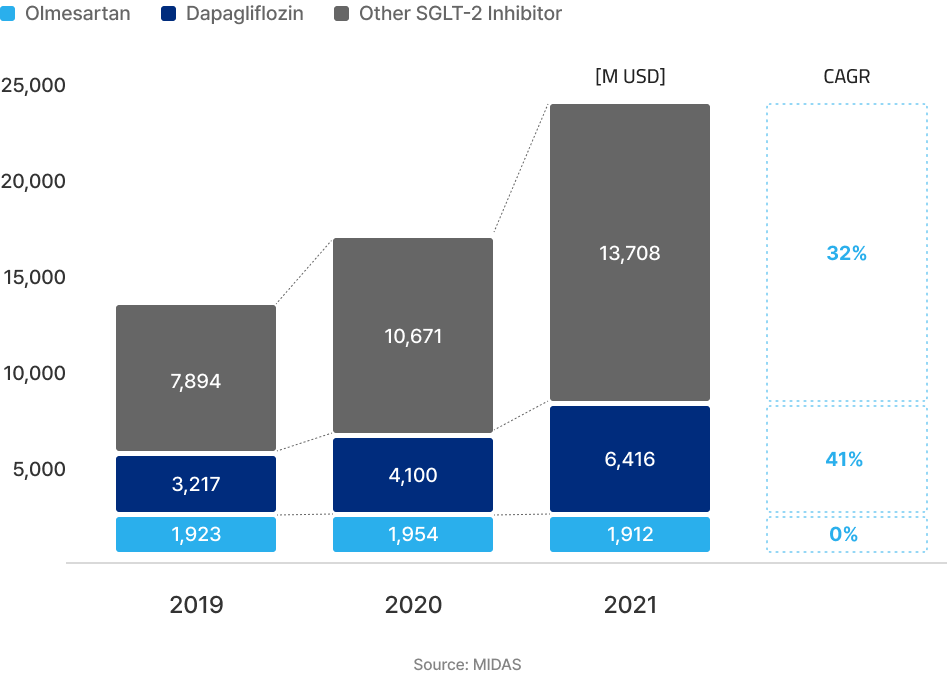
The market size for Olmesartan and Dapagliflozin combined is around 8.3 billion USD.
ATB-101 is expected to penetrate into other SGLT-2 inhibitors market with its improved medication compliance as a combination product.
Through Phase 1 clinical trials, it has been confirmed that there is no drug interaction when Dapagliflozin and Olmesartan are co-administered, and PK (Pharmacokinetic) equivalence has been established between the co-administration and combination therapy groups. Currently, Phase 3 clinical trials are ongoing at institutions such as Bundang Seoul National University Hospital, etc.
Overview of Clinical Trials
Primary Endpoints